Location:Home Page > Archive Archive
What is a supercapacitor? How is it different from conventional capacitors?
2023-04-04【Archive】
Supercapacitors, also known as electrochemical capacitors, electric double layer capacitors, gold capacitors, and farad capacitors, are electrochemical components developed in 1970s and 1980s to store energy using polarized electrolytes.

Unlike traditional chemical power sources, this power source has special properties between traditional capacitors and batteries. It is mainly based on electrical double layers and redox pseudocapacitive charges to store electrical energy. However, no chemical reaction occurs during its energy storage process, this energy storage process is reversible, and precisely because this supercapacitor can be repeatedly charged and discharged hundreds of thousands of times.
The exact design details of a supercapacitor depend on application and use of supercapacitor. These materials may vary slightly depending on manufacturer or specific application needs. Common to all supercapacitors is that they all contain a positive electrode, a negative electrode and a diaphragm between two electrodes, electrolyte fills two pores separated by two electrodes and the diaphragm.
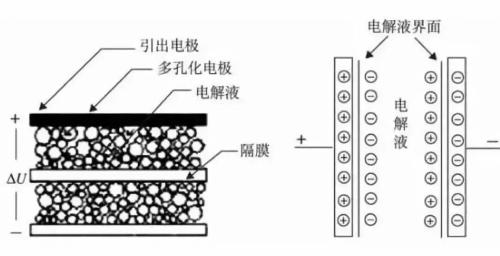
The figure shows structure of a supercapacitor consisting of a porous electrode material with a large specific surface area, a porous battery separator, and an electrolyte. The separator must satisfy conditions of highest possible ionic conductivity and lowest possible electronic conductivity, and is usually an electronic insulating material with a fibrous structure, such as a polypropylene film. The type of electrolyte is selected depending on material of electrode.

According to different energy storage mechanism, it can be divided into following two categories:
1.Electric double layer capacitance: results from collision of charges caused by directional arrangement of electrons or ions at electrode/solution interface. For an electrode/solution system, an electrical double layer will form at interface between electron-conducting electrode and ion-conducting electrolyte solution.
When an electric field is applied to two electrodes, anions and cations in solution migrate to positive and negative electrodes, respectively, forming a double electrical layer on surface of electrodes. positive and negative charges on electrodes are opposite to charges in solution. The charged ions are attracted to each other, stabilizing electrical double layer and creating a relatively stable potential difference between positive and negative electrodes.
At this time, for a certain electrode, same number of opposite sex ion charges as charges on electrode will be generated at a certain distance (scattering layer) so that it remains electrically neutral; when two poles are connected to external circuit, charge on electrode migrates to generate current in external circuit, and ions in solution migrate into solution to be electrically neutral. This is charging and discharging principle of electric double layer capacitor.
2. FaradFirst quasi-capacitance: Its theoretical model was first proposed by Conway, which is a two-dimensional or quasi-two-dimensional capacitor at electrode surface and near surface or in bulk phase. In three-dimensional space, electroactive substances undergo hypopotential deposition, and highly reversible chemical reactions of adsorption and desorption and redox reactions occur, as a result of which capacitance depends on charging potential of electrode.
For quasi-Faraday capacitance, charge accumulation process includes not only accumulation on electric double layer, but also includes a redox reaction between electrolyte ions and electrode active materials.
When ions (such as H+, OH-, K+, or Li+) in an electrolyte diffuse from solution to electrode/solution interface under action of an applied electric field, they will reach electrode surface via a redox reaction. at interface In bulk phase of active oxides, a large amount of charge accumulates in electrode.
When discharging, ions trapped in oxide are returned to electrolyte by reverse reaction of redox reaction described above, and accumulated charge is released through external circuit. It is charging and discharging mechanism of a quasi-Faraday-capacitor.
Advantages of supercapacitors:
1. Capacitance at farad level in a very small volume;
2. No need for special charge circuit and discharge control circuit;
3. Compared to a battery, overcharging and over-discharging will not adversely affect its lifespan;
4. In terms of environmental protection, it is a kind of green energy.
5. The supercapacitor can be welded, so there are no problems such as poor battery contact.
Disadvantages of supercapacitors:
1. Improper use may cause electrolyte leakage and other phenomena;
2. Compared with aluminum electrolytic capacitors, its internal resistance is relatively large, so it cannot be used in AC circuits;
Reason why supercapacitors are called "supercapacitors":
1. A supercapacitor can be thought of as two non-reactive porous electrode plates suspended in an electrolyte. When electricity is applied to plates, positive plate attracts negative ions in electrolyte, and negative plate attracts positive ions, in fact, formation of two capacitive storage layers separating positive ions near negative plate and negative ions near positive plate.
2. Supercapacitors store energy in separated charges. The larger area for accumulation of charges, denser separated charges, greater capacitance.
3. The area of a traditional capacitor is area of a flat conductor plate. In order to obtain a large capacitance, conductor material is rolled for a very long time, and sometimes a special organizational structure is used to increase its surface area. Traditional capacitors use insulating materials to separate two pole plates, typically plastic film, paper, etc. It is generally required that these materials be as thin as possible.
4. The area of the supercapacitor is based on porous carbon material. The porous structure of material allows its area to reach 2000 m2/g, and with some measures, a large surface area can be achieved. The distance over which supercapacitor charges diverge is determined by size of electrolyte ions that are attracted to charged electrodes. This distance (<10 µA) is less than that achievable with traditional film capacitor materials.
5. The huge surface area and very small charge separation distance make supercapacitors surprisingly large electrostatic capacitance compared to traditional capacitors, which is also their "super".

Supercapacitor Discharge Control:
The resistance of supercapacitor prevents it from discharging quickly. The time constant τ of supercapacitor is 1 ~ 2s, and it takes about 5 τ to fully discharge resistance-capacitance circuit, that is, it takes about 5 ~ 10s. for short-circuit discharge (due to special design of electrode, it actually takes hours to completely discharge residual charge).
Discharge control time:
Supercapacitors can charge and discharge quickly, peak current is limited only by their internal resistance, and even a short circuit is not fatal. Actually, it depends on size of capacitor bank. For load matching, a small bank can deliver 10A and a large bank can deliver 1000A. Another limiting factor in rate of discharge is heat, repeated discharge at a high rate will heat up capacitor and eventually open circuit.
Related
- What is a supercapacitor? How is it different from conventional capacitors?
- What is three anti-paint? How to use it correctly?
- The triode is used as a switch. You should know function of these capacitors which are commonly used.
- What is power supply ripple, how to measure their magnitude and how to suppress?
- What is purpose of connecting a polar capacitor and a non-polar capacitor in parallel?
- What is a delay scheme? Explanation of 6 Kinds of Delay Circuit Principles
- What is a magnetic sensor? The most common types of magnetic sensors and their applications
- What is drowning in gold? Why Shen Jin?
- About resistors, this is what you need to know
- What is difference between TVS tube and zener diode?
Hot Posts
 How to distinguish induction from leakage, we will teach you three tricks! Ordinary people can also learn super practical
How to distinguish induction from leakage, we will teach you three tricks! Ordinary people can also learn super practical
- What is drowning in gold? Why Shen Jin?
- This is a metaphor for EMI/EMS/EMC that can be understood at a glance.
- How many types of pads have you seen in PCB design?
- Summary of Common PCB Repair Techniques
- What is three anti-paint? How to use it correctly?
- Knowing these rules, you will not get confused looking at circuit diagram.
- How to make anti-interference PCB design?
- Can diodes do this?